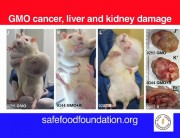
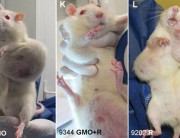
In defending his retraction of the Seralini paper, the journal’s editor makes a series of damaging admissions.
The Editor-in-Chief of Food and Chemical Toxicology, A. Wallace Hayes, has come under severe criticism after retracting from his journal the paper by Seralini at al on the long term toxicity of Roundup and a Roundup-tolerant GM maize. He has now published a defence of the retraction, which we reproduce below.
Here are some of the major problems with Hayes’ defence of his retraction decision:
1. Hayes states that Dr Seralini’s “claim (ie, conclusion) that Roundup Ready maize NK603 and/or the Roundup herbicide have a link to cancer is unreliable” because the data on this are “inconclusive”. He goes on to say that “Dr. Séralini deserves the benefit of the doubt that this unreliable conclusion was reached in honest error. The review of the data made it clear that there was no misconduct. However, to be very clear, it is the entire paper, with the claim that there is a definitive link between GMO and cancer that is being retracted.”
BUT there is no claim or conclusion in the paper “that Roundup Ready maize NK603 and/or the Roundup herbicide have a link to cancer”; nor does the “entire paper” “claim that there is a definitive link between GMO and cancer”. IN FACT, THE ENTIRE SERALINI PAPER DOES NOT MENTION THE WORD “CANCER” ANYWHERE!
This was a long term toxicity study – the clue is in the title: “Long term toxicity of a Roundup herbicide and a Roundup-tolerant genetically modified maize”. Seralini did not set out to study carcinogenicity, or to conduct a carcinogenicity study. He has said he did not expect to find evidence of carcinogenicity, but he did find evidence of tumours in the treatment groups during the study, which he reported, as he should. Tumours unexpectedly found in a chronic tox study MUST be reported according to OECD452 chronic toxicity protocol (“lesions”), so Seralini had to note them in the paper. But he noted them without drawing definitive conclusions or extrapolating his findings to human carcinogenicity.
His toxicity study has now been retracted on the grounds of claiming “a definitive link between GMO and cancer”, which it nowhere makes. So, did Hayes actually read the Seralini paper before retracting it? Because if he did, how come he doesn’t know what the paper says?
Hayes not having read the paper is, of course, the charitable interpretation of his actions, because if he is familiar with the paper’s contents it might be hard not to conclude that he has deliberately misrepresented them.
2. Hayes defends the study done by Monsanto (Hammond et al., 2004) which his journal also published, but which it has not retracted. The Monsanto study, Hayes says, included 20 rats per sex per group, whereas Seralini et al only included 10 rats per sex per group. But what Hayes fails to acknowledge is that the Monsanto study only *analysed* 10 rats per group (i.e. 50% of the animals) for blood and urine chemistry (the main chronic toxicity endpoints), meaning selection bias was introduced. Seralini used 10 per sex per group in total, so just as many animals per group were analysed without any possibility of selection bias. Thus, Seralini’s methodology was more rigorous than that of Monsanto’s, while generating just as much data per group.
3. Hayes admits the chronic toxicity findings are justified by a study of the size that Seralini et al carried out. In that case, why not simply ask Seralini to publish a clarification to his paper to the effect that the “chronic toxicity findings statistics are significant; tumour findings need followup”, if that is what is felt to be necessary? That is the correct approach according to the Committee on Publication Ethics (COPE) guidelines that Hayes claims to be abiding by. There is nothing in the COPE guidelines that justifies or requires the retraction of the whole paper.
4. Hayes argues that his appoinmtment of a former Monsanto editor (Richard Goodman) as an Associate Editor at his journal (FCT) with particular responsibility for biotechnology, could not have been a biasing factor in the (re)review of the Seralini paper that led to its retraction. Hayes claims that part way through the (re)review of Seralini’s data, Seralini requested that Goodman not take part in this process and that Hayes readily agreed to this.
BUT Hayes also admits that, “Professor Goodman, along with all other members of the editorial board was involved in initial discussions of the Séralini paper and the request to view raw data.” The fact that this former Monsanto employee was involved in discussion of the (re)review of the Seralini paper from the start, and up until Seralini directly requested that this stop, means that he could not only have influenced the discussion but even more crucially the selection of the review panel members. This is especially the case because we know Goodman was appointed by Hayes in the light of his criticisms of the Seralini paper and as having particular expertise in this area, all of which suggests that Hayes would have been likely to take his views into account.
Food and Chemical Toxicology Editor-in-Chief, A. Wallace Hayes, Publishes Response to Letters to the Editors
Cambridge, MA, December 10, 2013
The following statement will be published in the journal, Food and Chemical Toxicology, alongside a selection of letters to the editors regarding the decision to retract the paper by Séralini et al.(Séralini et al., 2012).
In November 2012, this journal published an article titled “Long term toxicity of a Roundup herbicide and a Roundup-tolerant genetically modified maize,” by Séralini et al.(Séralini et al., 2012). The publication of this article caused quite a stir in the media, as well as in the scientific community. The journal received many letters expressing concerns about the validity of the findings. A careful and time-consuming analysis found that the data were inconclusive, and therefore the conclusions described in the article were unreliable. Accordingly, the article was retracted. Since the public announcement of the retraction, the journal has received many letters to the editor; a selection of these letters will be published, along with this response to those letters. Many of these letters expressed concerns about the decision making process behind this action, particularly what role (if any) current or former Monsanto employees played, whether or not COPE guidelines were followed, and if the journal was also considering retraction of a similar paper by Hammond et al.(Hammond et al., 2004). The answers to these questions are below.
The membership of the editorial board is composed of academic, government, and industrial scientists. Contrary to what has been suggested by some, the appointment of Professor Richard Goodman, University of Nebraska, as an Associate Editor was not influenced by Monsanto or any other party. Members of the editorial board are chosen based on their expertise as scientists. It is the goal of this journal to have a variety of different viewpoints. In this case, as in other cases, I as Editor-in-Chief listened to as wide and diverse a set of expertise as possible. To wit, Professor Goodman, along with all other members of the editorial board was involved in initial discussions of the Séralini paper and the request to view raw data. When the request was made to Dr. Séralini to review the raw data, the journal suggested to Dr. Séralini that all parties involved sign a confidentiality agreement. This confidentiality agreement was designed to protect Dr. Séralini and his data so that it was (A) not viewed by anyone he did not want to view his data and (B) that it would not go beyond the people he agreed would review the raw data. Not initially, but during the process, Dr. Séralini made a direct request that Professor Goodman be excluded, and we at FCT readily and quickly agreed. It is understandable that Dr. Goodman’s involvement, however small, might be cause for concern for some. However, the decision to retract the paper was mine alone, made by me exclusively and not by a vote of the editorial board. Further, when Dr. Séralini asked for Dr. Goodman’s involvement to stop, I agreed, fully and promptly.
The Monsanto Company did write a letter to the editor regarding this article, and it was published along with a number of other letters to the editor (Hammond et al., 2013); neither the company nor any of their scientists put any pressure on the Editor in Chief regarding this matter.
A second concern that has been raised is whether this retraction follows the COPE guidelines. The COPE guidelines were consulted when making this decision. According to the COPE guidelines, “Journal editors should consider retracting a publication if… they have clear evidence that the findings are unreliable, either as a result of misconduct (e.g. data fabrication) or honest error (e.g. miscalculation or experimental error).”(COPE, 2009). The retraction statement could have been clearer, and should have referred to the relevant COPE guidelines. The data are inconclusive, therefore the claim (ie, conclusion) that Roundup Ready maize NK603 and/or the Roundup herbicide have a link to cancer is unreliable. Dr. Séralini deserves the benefit of the doubt that this unreliable conclusion was reached in honest error. The review of the data made it clear that there was no misconduct. However, to be very clear, it is the entire paper, with the claim that there is a definitive link between GMO and cancer that is being retracted. Dr. Séralini has been very vocal that he believes his conclusions are correct. In our analysis, his conclusions cannot be claimed from the data presented in this article.
At this point it is very important to state that the retraction does not reflect or impact the journal’s view on GMOs or associated organizations. Our journal would, in fact, very much welcome the opportunity to review follow-up studies that have a greater sample size, a fine-tuned method, and proper controls. We are also actively searching and recruiting people to provide a balance view on this topic to serve on the editorial board.
Finally, the letters post-retraction have questioned whether an earlier study done by Monsanto received different treatment from our journal. This article was published in 2004, well before I became Editor-in-Chief. However, I take the issue seriously and have reviewed this paper in detail. The Hammond et al. article was a 13 week feeding study performed in rats feed grain from Roundup Ready corn which is tolerant to the herbicide glyphosate (Hammond et al., 2004). The authors reported the following: “Purina TestDiets formulated Roundup Ready corn grain into rodent diets at levels of 11 and 33% (w/w). The responses of rats fed diets containing Roundup Ready corn grain were compared to that of rats fed diets containing non-transgenic grain (controls). All diets were nutritionally balanced and conformed to Purina Mills, Inc. specifications for Certified Lab Diet 5002. There were 400 rats in the study divided into 10 groups of 20 rats/sex/group. Overall health, body weight, food consumption, clinical pathology parameters (hematology, blood chemistry, urinalysis), organ weights, gross and microscopic appearance of tissues were comparable between groups fed diets containing Roundup Ready and control corn grain… This study complements extensive agronomic, compositional and farm animal feeding studies with Roundup Ready corn grain, confirming it is as safe and nutritious as existing commercial corn hybrids.” The authors also stated “The study design was adapted from OECD Guideline No. 408 (1981) and the study was reported to have been conducted in general compliance with OECD Good Laboratory Practice (GLP) guidelines at the Metabolism and Safety Evaluation-Newstead, toxicology laboratory.”
In accordance with OECD Guideline No. 408 (OECD, 2009a), the Hammond et al. study was limited to 90 days following and used 20 rats/sex/group, and was conducted in general compliance with OECD Good Laboratory Practice (GLP) guidelines, as previously stated. The Séralini et al. study ran for two (2) years with only 10 rats/sex/group and was reported to be done in a GLP environment according to OECD guidelines (which guideline is not explicitly stated in the paper). Séralini et al. state that they had “had no reason to settle at first for a carcinogenesis protocol using 50 rats per group,” as recommended in OECD Nos. 451 and 453 (guidelines for Carcinogenicity Studies and Combined Chronic Toxicity/Carcinogenicity Studies, respectively)(OECD, 2009a; OECD, 2009b), and instead seem to have opted for 10 rats/sex/group as recommended by OECD No. 408 (guidelines for Repeated Dose 90-day Oral Toxicity Study in Rodents). While the number of animals used may have been sufficient to reach conclusions regarding oral toxicity, it proved insufficient for conclusions related to the carcinogenicity of the test substances.
In conclusion, FCT has retracted this article because our thorough investigations revealed that its methods were scientifically flawed. The low number of animals, and the strain selected, rendered the conclusions unreliable. No definitive conclusions could be drawn from the inconclusive data. Therefore, in accordance with both the COPE guidelines and the journal policy, it was necessary to take this action of retracting the article.
A. Wallace Hayes, PhD, DABT, FATS, FIBiol, FACFE, ERT
Registered Toxicologist (France and EUROTOX registries)
Harvard School of Public Health
Editor-in-Chief, Food and Chemical Toxicology
References
COPE, 2009. Retraction guidelines.
Hammond, B., Dudek, R., Lemen, J., Nemeth, M., 2004. Results of a 13 week safety assurance study with rats fed grain from glyphosate tolerant corn. Food Chem. Toxicol. 42, 1003–14.
Hammond, B., Goldstein, D. a, Saltmiras, D., 2013. Response to original research article, in press, corrected proof, “‘Long term toxicity of a Roundup herbicide and a Roundup-tolerant genetically modified maize’”. Food Chem. Toxicol. 53, 459–64.
OECD, 2009a. OECD Guideline for the Testing of Chemicals.
OECD, 2009b. Combined Chronic Toxicity \ Carcinogenicity Studies.
Séralini, G.-E., Clair, E., Mesnage, R., Gress, S., Defarge, N., Malatesta, M., Hennequin, D., de Vendômois, J.S., 2012. Long term toxicity of a Roundup herbicide and a Roundup-tolerant genetically modified maize. Food Chem. Toxicol. 50, 4221–31.
# # #
About Elsevier
Elsevier is a world-leading provider of scientific, technical and medical information products and services. The company works in partnership with the global science and health communities to publish more than 2,000 journals, including The Lancet and Cell, and close to 20,000 book titles, including major reference works from Mosby and Saunders. Elsevier’s online solutions include ScienceDirect, Scopus, SciVal, Reaxys, ClinicalKey and Mosby’s Suite, which enhance the productivity of science and health professionals, helping research and health care institutions deliver better outcomes more cost-effectively.
A global business headquartered in Amsterdam, Elsevier employs 7,000 people worldwide. The company is part of Reed Elsevier Group plc, a world leading provider of professional information solutions. The group employs more than 30,000 people, including more than 15,000 in North America. Reed Elsevier Group plc is owned equally by two parent companies, Reed Elsevier PLC and Reed Elsevier NV. Their shares are traded on the London, Amsterdam and New York Stock Exchanges using the following ticker symbols: London: REL; Amsterdam: REN; New York: RUK and ENL.
Media contact
Meghan Jendrysik
Elsevier
+1 617 397 2845
m.jendrysik@elsevier.com
newsroom@elsevier.com