We cautiously welcome EFSA’s release of the data it used to approve NK603 maize, though we await scientists’ views as to whether it is useful and usable data.
However, Seralini has also asked for the data on glyphosate to be made public and this is another matter altogether. Thus far several national governments in EU member states have refused to make this data public in response to requests from Pesticide Action Network Europe.
It seems the data on glyphosate is kept in a bunker somewhere in Germany and no one is allowed to see it. It’s our impression that neither the Commission nor EFSA has seen it either, albeit they have not hesitated to issue reassuring statements about the safety of the herbicide.
If this data is ever released, we predict that glyphosate would be doomed. Independent scientists would see evidence of birth defects and cancer in the experimental animals in industry’s own studies – as revealed by Earth Open Source’s examinations of the German government report on the secret industry studies.
Scientists would also object to the fact that the industry tests were on glyphosate alone, not the commercial formulations of the herbicide as used. So these tests are not valid evidence to support the safety of the commercial formulations.
—
GM: EFSA makes public the data on the NK603 maize denounced by Seralini
AFP, via Romandie.com, 14 Jan 2013
English translation by GMWatch
BRUSSELS – The European Food Safety Authority (EFSA) posted Monday all the data it used to give its opinion in favour of authorizing the commercialization of GM maize NK603, denounced by French scientist Gilles-Eric Seralini.
The initiative launched today aims to make the data used in the risk assessment publicly available, said the Director General of the agency, Catherine Geslain-Laneelle, in a statement.
Through this program, EFSA will help scientists from different fields of expertise to develop research to ultimately enrich the body of scientific literature and offer valuable new perspectives that can be integrated into the risk assessment, she added.
For her, it will further reinforce the conclusions of [EFSA] opinions aimed at protecting public health and will also strengthen trust in the work of EFSA.
Opening the file posted on the EFSA website takes twenty minutes, AFP noted.
EFSA had already made these data available to scientists in response to the criticisms of French scientist Gilles-Eric Seralini, author of a study on the toxicity of NK603 which was rejected by the agency.
The French researcher refused to transmit the information [to EFSA] to fill in gaps in his study. “We expect them (EFSA) to provide the elements that allowed them to authorize the GMO and the pesticide,” he declared.
The evaluation process for applications for authorization of cultivation and marketing of GMOs is conducted in four phases: consultation of EFSA on health hazards, request for authorization by the the member states on the basis of a favorable opinion from EFSA, and appeal procedure if no qualified majority emerges from the member states. Finally, if this situation continues, the final decision rests with the European Commission.
To date, EFSA has never issued a negative opinion and no qualified majority was ever found among states to prohibit or authorize a GMO.
Two GMOs have been approved for cultivation based on the decision of the European Commission – the potato Amflora from the German company BASF and Monsanto’s MON810 maize. Fifty others, including NK603 maize, have been authorized for animal feed and human food.
Comment by GMWatch
14 January 2013
















































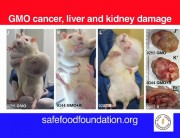

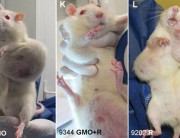

















We MUST have access to data on the safety of glyphosate (roundup), especially since the reports of major human problems in S America