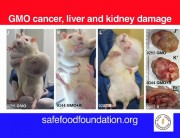
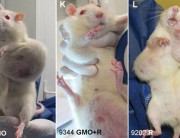
Today the independent scientific research organisation CRIIGEN announced its formal withdrawal from the French government’s Risk’OGM project. Risk’OGM was intended to follow up the findings of CRIIGEN researchers, led by Prof Gilles-Eric Séralini, that GM maize NK603 and the Roundup herbicide it is engineered to tolerate were toxic to rats fed over the long term.
https://www.gmoseralini.org/
Claire Robinson
GMWatch, 28 May 2014
In April this year, France’s ministry of the environment announced that it would allocate a total budget of 3.7 million Euros to the Risk’OGM project. This is higher than Séralini’s already considerable budget of 3 million Euros.
http://www.developpement-
Accordingly, the French study should have been more detailed than Séralini’s and should certainly have been long-term (2 years in rats).
But earlier this year, Earth Open Source learned from ANSES that the French authorities had decided to fund a study of only 6 months or less. The study, entitled “Improved predictability of sub-chronic GMO toxicity by identification of early biomarkers of toxicity” ANSES explained, “aims at improving the 90 day study in order to better and earlier detect potential side effects. This 6-month study will be carried out using various kinds of ‘omics’ analyses.”
It is unfortunate that the French government has turned its back on the long-term study that could have answered questions about the safety of NK603 and Roundup more definitively. It is incomprehensible that it has reduced a potential long-term study of 2 years down to a virtually useless 6 months.
“Omics” analyses can indeed add valuable information to our knowledge of GMO and pesticide health effects, but only in retrospect, after serious diseases like tumours, cancers or organ damage have already appeared. Such “omics” analyses can then look more deeply at pathways and biological processes through which the diseases arose.
If initial signs of potential long-term toxicity are identified through “omics” analyses in a short or medium-term study of only 3-6 months but the study is not extended to see whether any serious disease actually appears, then those initial signs can be easily dismissed as “not biologically relevant”.
This is a favourite tactic of GMO proponents in industry and government alike and in fact it was used by the European Food Safety Authority (EFSA) and Monsanto to dismiss initial signs of toxicity in Monsanto’s 90-day study on NK603 maize. This dismissal led to the maize’s approval.
Therefore the study led by ANSES is likely to be a waste of public money. It will enable industry and pro-GMO lobbyists to continue to pretend that GMOs are safe. It fulfils the number one rule of the GMO industry and its lobbyists when it comes to scientific studies looking at GMOs and pesticide risks: Don’t look, don’t find.
The EU has also announced a two-year carcinogenicity study on NK603 maize to follow up the Séralini study findings. But no protocol has been published and we are told that the group that won the bid has industry involvement.
There are ways to design a carcinogenicity study and not find anything. In my view, the most likely is that no attempt will be made to exclude GMOs (apart from the NK603 maize that is the focus of the test) and toxic residues of pesticides from the control diets. Thus both treated and control groups of rats will be eating GMOs and pesticides and any toxic effects from the GMO under test will be hidden in the resulting “data noise”.
It’s a testament to the strength of the Séralini 2012 study that even governments are frightened of carrying out a true replication and extension of the research. What can they be frightened of?