Summary answer:
No study is perfect, but Séralini’s is far stronger than the 90-day studies carried out by industry for regulatory authorization of GM foods. It is unacceptable that these studies are accepted as proof of safety for GM foods but that Séralini’s study, which extended these industry studies and found risk, is dismissed.
Detailed answer:
No scientific study is perfect – all have flaws and limitations. But Séralini’s is far stronger, in terms of study length, parameters examined, and carefulness of design (which enabled distinction between effects of the genetic modification and the herbicide it is grown with) than the 90-day studies carried out by industry for regulatory authorization of GM foods. Criticisms of Séralini’s study only highlight the weaknesses of these industry studies.
A deficiency in the presentation of the results in Séralini’s publication is that it does not include the raw data for the male liver-kidney parameter measurements. The authors say in the text that most males died prematurely from severe damage to these organs, summarised in Table 2, but they do not show the actual data on which these conclusions are based. Table 2 shows only a summary of the disorders that the animals suffered in a given test group. It does not show the actual measurements of parameters underlying the categorisation shown in Table 2. Even Table 3 shows only percentage of variation between treatments and controls and not the actual measurements underlying the percentage variations listed.1
However, Séralini and colleagues state in their rebuttal to criticisms of this study that all data will be made available in future publications.2
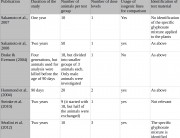
Furthermore, this weakness of presentation in this current publication must be judged against the many serious weaknesses in industry studies performed on GMOs for regulatory authorizations. For example, Monsanto’s 90-day feeding trial on NK603 maize,3 which EFSA accepted as proof of safety, has the following weaknesses:
- Six irrelevant “reference” control diets were introduced. These only served to introduce “data noise”, which largely masked the effects of the genetic modification. In fact, significant differences were revealed in liver and kidney measurements, but these were masked by the wide range of variables introduced by the six irrelevant “reference” diets. This is scientific sleight-of-hand.
- The rats were only followed over 90 days, a relatively short period equivalent to around 7-9 human years.4 This is inadequare to assess health risks to humans, who can be expected to eat a GM food over a lifetime.
- Statistically significant differences were found but were dismissed as not treatment-related or not biologically meaningful, without scientific justification. The study period was not extended to see if these differences were in fact biologically meaningful.
- Blood and urine samples were analyzed from only ten rats out of groups of 20. Yet organ weights were measured for all 20 rats per group. So the Monsanto researchers selected ten rats per sex out of 20 to analyze. What were the selection criteria? Did they choose those whose organ weights were the healthiest? Or did they analyze all 20 rats from each group and select the ten most favourable measurements to report? The method of selecting well may have been objective and random, but there is no way of knowing, and apparently, EFSA does not care. This practice invalidates the study as it introduces a scientifically unacceptable selection variable.
- Fewer parameters were measured than in Séralini’s study, and less often.
- Fewer doses of the maize were administered than in Séralini’s study (two, to Séralini’s three). The Monsanto researchers tried to draw a dose-response conclusion from feeding just two doses (11% and 33% maize), but a minimum of three doses are necessary to draw a valid dose-response conclusion, since with only two points you can draw a straight line between them regardless of how they relate to one another. Three doses are required by the OECD 408 protocol on which Monsanto researchers base their studies; it is unclear why Monsanto 90-day studies habitually use only two.
The effects of Roundup, the herbicide with which NK603 maize is grown, were not tested separately from the NK603 maize. So the effects found could have been due to the herbicide or the genetic modification, or a combination of both.
References:
1. Séralini GE, Clair E, Mesnage R, et al. Long term toxicity of a Roundup herbicide and a Roundup-tolerant genetically modified maize. Food and Chemical Toxicology. November 2012; 50(11): 4221-4231.
2. Séralini GE, Mesnage R, Defarge N, et al. Answers to critics: Why there is a long term toxicity due to NK603 Roundup-tolerant genetically modified maize and to a Roundup herbicide. Food and Chemical Toxicology. 9 November 2012.
3. Hammond B, Dudek R, Lemen J, Nemeth M. Results of a 13 week safety assurance study with rats fed grain from glyphosate tolerant corn. Food Chem Toxicol. Jun 2004; 42(6): 1003-1014.
4. Soffritti M, Belpoggi F, Degli Esposti D. Cancer prevention: The lesson from the lab. In: Biasco G, Tanneberger S, eds. Cancer Medicine at the Dawn of the 21st Century: The view from Bologna. Bologna: Bononia University Press; 2006:49–64.
Sources of criticism:
Science Media Centre “experts”
http://www.sciencemediacentre.org/expert-reaction-to-gm-maize-causing-tumours-in-rats/
European Food Safety Authority
http://www.efsa.europa.eu/en/efsajournal/pub/2910.htm
http://www.efsa.europa.eu/en/efsajournal/pub/2986.htm