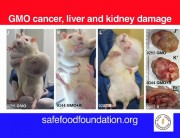
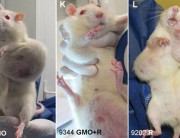
Some government agencies were quick to condemn Séralini’s study. But they are not disinterested parties, since they have been responsible for authorizations of GM NK603 maize and Roundup. For example, the European Food Safety Authority (EFSA), the French food safety agency ANSES, and the French Haut Conseil des Biotechnologies (HCB), all of which rejected Séralini’s findings, have previously issued favorable opinions about the safety of NK603 maize that led to authorizations at the EU level and at the national level in France. 1
As Member of the European Parliament Corinne Lepage said of EFSA’s rejection of Séralini’s study: “To recognize the validity of the study would be to cut the branch on which the agency has sat for years, since all its opinions on GMOs were positive.” 1
The German federal institute for risk assessment, BfR, also rejected Séralini’s findings. 2 But Germany’s government regulatory authorities are responsible for the EU authorization of glyphosate, the main chemical ingredient of Roundup. 3 So in this case, too, the critic is not a disinterested party.
There are questions too about whether some of the impressive-sounding official reports condemning Séralini’s study are genuinely representative of their organizations.
A report purportedly from six French scientific academies was exposed as biased and unrepresentative by the academies’ statistics expert, Paul Deheuvels. According to Deheuvels, the academies’ membership as a whole was not consulted about the report, which was written by an anonymous group of experts “convened in an emergency, we do not know by whom, no one knows how, with a total lack of transparency in the selection of its members, and on the basis of two representatives from each academy”. 4
As the academies’ report focused heavily on the statistical aspects of Séralini’s study, it would have been natural for Deheuvels to be consulted – but he was not. 4
Deheuvels’s verdict on the report was supported by 140 French scientists in an open letter to Le Monde newspaper. The scientists wrote:
“The fact that a group of a dozen people claiming to represent six academies [of science] have agreed a joint statement [condemning Séralini] without debate is contrary to the normal functioning of these institutions and brings into question the image of science and technology.” 5
As for EFSA’s response to Séralini’s study, it was coordinated in a rushed teleconference with the participation of only four member states (Germany, Belgium, Netherlands, and France) out of a total of 27 – hardly a representative sample. A stated aim of the conference, according to the EFSA representative, was to “avoid divergence” of opinion about the study. The Belgian representative even offered to pressure the editor of Food and Chemical Toxicology, the journal that published Séralini’s study, to retract it. 6
Interestingly, two months after the publication of Séralini’s study, the online magazine EU Food Policy reported that EFSA was in talks with the EU Commission about potentially arranging studies to test long-term methodologies for testing GM foods. This is a breakthrough because it is the first time that EFSA has ever admitted that long-term studies are desirable. But there were no plans to test the maize thrown into question by Séralini’s study, NK603. Instead, another Monsanto maize, MON810, was put forward. EFSA was reported as offering to help the Commission design such studies, though as long as the agency refuses to concede any validity in Séralini’s study, its potential role in this project remains deeply suspect. 7
References:
1. Lepage C. OGM: l’EFSA a manqué à une déontologie élémentaire [GMOs: EFSA breaches basic ethical code]. Le Nouvel Observateur. 7 October 2012. http://bit.ly/QWjizy
2. Bundesinstitut für Risikobewertung (BfR). A study of the University of Caen neither constitutes a reason for a re-evaluation of genetically modified NK603 maize nor does it affect the renewal of the glyphosate approval. 1 October 2012. http://bit.ly/Sz6lRI
3. Antoniou M, Habib MEM, Howard CV, et al. Teratogenic effects of glyphosate-based herbicides: Divergence of regulatory decisions from scientific evidence. J Environ Anal Toxicol. 2012.
4. Deheuvels P. L’étude de Séralini sur les OGM, pomme de discorde à l’Académie des sciences [The Seralini GMO study – A bone of contention at the Academy of Sciences]. Le Nouvel Observateur. 19 October 2012. http://leplus.nouvelobs.com/contribution/661194-l-etude-de-seralini-sur-les-ogm-pomme-de-discorde-a-l-academie-des-sciences.html
5. Andalo C, Chercheuse AHS, Atlan A, Auclair D, Austerlitz F, Barot S. Science et conscience [Science and conscience]. Le Monde. 14 November 2012. http://www.lemonde.fr/idees/article/2012/11/14/science-et-conscience_1790174_3232.html
6. European Food Safety Authority (EFSA) Emerging Risks Unit. Teleconference with member states on Seralini et al study 28 September 2012, 10.30-12.30, 1st meeting report. 28 September 2012. https://zoek.officielebekendmakingen.nl/blg-187499.html
7. Trollope, K. Commission and EFSA agree need for two-year GMO feeding studies. EU Food Policy. 17 December 2012.