- 41 scientists and experts demand reinstatement of the study
- Scientists around the world invited to add their signatures
Press release, endsciencecensorship.org, 29 January 2014
SCIENTISTS from around the world have united to condemn the retraction[1,2] by the editor of the Elsevier journal Food and Chemical Toxicology (FCT) of the pioneering study on genetically modified (GM) maize and Roundup herbicide, conducted by Prof Gilles-Eric Séralini and his team at the University of Caen, France.
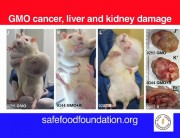
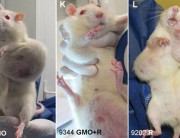
The study found that rats fed the GM maize and/or low levels of Roundup in their diet suffered severe organ damage, particularly to the liver, kidneys and pituitary gland. Additional unexpected observations were higher rates of large tumours and mortality in most treatment groups.[3]
A group of 41 scientists and biosafety experts have signed a public statement condemning the retraction as an apparent “act of scientific censorship” and demanding that the journal reinstate the study, which they say contains findings of potentially critical importance to public health.[4]
The group is inviting scientists to sign on to the statement, which is hosted at the new website, endsciencecensorship.org.
On the website, several of the signatory scientists explain the study and its findings for the non-specialist, as well as providing a detailed examination of the reasons given for the retraction.
The public is invited to support the initiative by demanding that the German government release the industry toxicology studies on glyphosate, the main chemical ingredient of Roundup. Though these studies form the basis of the EU approval of all glyphosate herbicides, they are kept secret and are held by the German government.[5]
Background
[Comprehensive references to sources are at endsciencecensorship.org]
Séralini’s study passed peer review with minor revisions and was published by FCT in September 2012. The paper was subjected to a sustained campaign of attack by pro-GM scientists, many of whom had undisclosed links to the GM crop industry or with government agencies that had previously approved this or other GM crops as safe.
FCT’s editor, Dr A. Wallace Hayes, retracted the paper over a year after it was published. The decision came after a nontransparent, second review by a panel of unnamed persons of unknown professional competence and with undisclosed potential conflicts of interest.
According to the Committee on Publication Ethics (COPE), of which FCT is a member, retraction of a paper is reserved for cases of misconduct, error, redundant publication or plagiarism, and unethical research.
None of these criteria apply to the Séralini paper, as Dr Hayes conceded. Dr Hayes stated that the retraction was solely based on the “inconclusive” nature of the tumours and mortality outcomes, based on the relatively low number of animals and the strain of rat used,[6] which is reportedly prone to spontaneous tumours. Dr Hayes wrote: “No definitive conclusions could be drawn from the inconclusive data”.[7]
However, the scientists say that the lack of “definitive conclusions” is an invalid and unprecedented reason for retracting a study, since many scientific papers contain inconclusive findings.
In addition, the main findings of the Séralini study were severe organ toxicity in the animals fed GM maize and/or Roundup. Yet these findings, which are statistically significant and as conclusive as it is possible for such results to be, have also been erased, due to the presence of some alleged “inconclusive” findings in the paper.
Just months before the retraction was announced, Dr Richard E. Goodman, a former Monsanto scientist, was appointed to a newly created editorial position in biotechnology at FCT. Soon after Goodman’s appointment, a second paper challenging the safety of GM crop technology was withdrawn from FCT[8] (though it was immediately re-published in another journal).[9]
In a separate initiative, over 860 scientists worldwide have condemned the retraction in an open letter to FCT and have announced that they will boycott Elsevier journals.[10]
Scientists’ comments on the retraction
Dr Angelika Hilbeck, senior scientist, Swiss Federal Institute of Technology, Institute of Integrative Biology IBZ, Zurich, Switzerland; Chair, European Network of Scientists for Social and Environmental Responsibility (ENSSER), said:
“This retraction lacks any scientific basis. If the lack of ‘definitive conclusions’ were a valid reason for retraction, our libraries would be almost empty, as I have yet to see a study that yielded results of ‘definitive’ conclusiveness.
“In fact, the surprising revelation of FCT’s highly irregular post-publication evaluation process is that Séralini’s study would appear to be flawless. In response to massive, orchestrated pressure from industry-aligned circles, a secret group of unknown people was set up with the sole aim of finding weaknesses in the study to allow it to be shot down and retracted (we have no verifiable evidence that these people were experts in the relevant fields of science or in anything, for that matter). Yet after trying hard for one full year, all they could come up with was a lack of ‘definitive conclusions’.
“To me, this is a confirmation of the quality of the study and the integrity of the researchers, as very few studies would pass such an extraordinary evaluation process.”
Dr Michael Antoniou, a molecular geneticist based at a leading London medical school, said:
“Based on the science presented in the Séralini paper, it is extremely difficult to see any cause for retraction, especially since the journal’s editor, Dr A. Wallace Hayes, only highlighted two of several aspects of the paper – the tumours and mortality rates – as ‘inconclusive’.
“This suggests that even in the editor’s view, the bulk of the findings – the severe organ toxicity in rats fed GM maize and low levels of Roundup – must be valid. To retract the whole paper based on the perceived inconclusiveness of just a proportion of the data presented is scientifically untenable.
“In deciding to retract the paper, the editor is not being true to the science. I conclude that the retraction must be due to other, non-scientific reasons.”
Dr David Schubert, professor, Salk Institute for Biological Studies, CA, USA, said:
“The editor of the journal claimed that the reason for retraction of the Séralini study was that ‘no definitive conclusions can be reached’. As a scientist, I can assure you that if this were a valid reason for retracting a publication, a large fraction of the scientific literature would not exist.
“Before the retraction, a former Monsanto scientist was brought into the journal as biotechnology editor. Therefore, a combination of intense pressure from scientists and a new pro-industry editor led to the elimination of an exceptionally important study.
“I am convinced that there is significant evidence, like that presented by Séralini, that some GM foods are hazardous to human health. In order for data supporting this possibility to enter public discourse, scientists must place their ethical responsibilities above corporate profits and cease their continual assault on the science relating to GM safety. The protection of scientists’ right to publish their findings without censorship or retribution must be preserved.”
Notes
1. Hayes AW (2013). Elsevier (2013). Elsevier announces article retraction from Journal Food and Chemical Toxicology. 28 November.http://endsciencecensorship.us3.list-manage1.com/track/click?u=d6862ffb965b93d2616631e16&id=0ea9793ac8&e=477dd18fb5
2. Hayes AW (2013). Letter to Prof GE Séralini. 19 Nov. Available at: http://endsciencecensorship.us3.list-manage.com/track/click?u=d6862ffb965b93d2616631e16&id=9fab9cea2e&e=477dd18fb5
3. Séralini GE et al (2012). Long term toxicity of a Roundup herbicide and a Roundup-tolerant genetically modified maize. Food and Chemical Toxicology 50(11): 4221-4231 [WITHDRAWN]. The study is available here: http://endsciencecensorship.us3.list-manage2.com/track/click?u=d6862ffb965b93d2616631e16&id=13c63d0f96&e=477dd18fb5
4. www.endsciencecensorship.org
5. Public petition at: http://endsciencecensorship.us3.list-manage2.com/track/click?u=d6862ffb965b93d2616631e16&id=820c96d917&e=477dd18fb5 Prof Séralini has already released the raw data for his study to the editor of FCT, who reported that he found nothing “incorrect” about the results and no evidence of fraud (http://endsciencecensorship.us3.list-manage.com/track/click?u=d6862ffb965b93d2616631e16&id=ffbf9323c9&e=477dd18fb5). Prof Séralini has also deposited his raw data with a notary, for public release as soon as government authorities make public all industry data on pesticides and GMOs. Industry data on GMOs is already available on request from EFSA, though not in an analyzable form (Excel/Word).
6. Hayes AW (2013). Letter to Professor GE Séralini. 19 Nov. Available at: http://endsciencecensorship.us3.list-manage.com/track/click?u=d6862ffb965b93d2616631e16&id=7027ea446c&e=477dd18fb5
7. Hayes AW (2013). Food and Chemical Toxicology editor-in-chief, A. Wallace Hayes, publishes response to Letters to the Editors. 10 Dec.http://endsciencecensorship.us3.list-manage.com/track/click?u=d6862ffb965b93d2616631e16&id=1670a2c5c2&e=477dd18fb5
8. Mezzomo BP et al (2012). WITHDRAWN: Effects of oral administration of Bacillus thuringiensis as spore-crystal strains Cry1Aa, Cry1Ab, Cry1Ac or Cry2Aa on hematologic and genotoxic endpoints of Swiss albino mice. Food Chem Toxicol.
9. Mezzomo BP et al (2013). Hematotoxicity of Bacillus thuringiensis as spore-crystal strains Cry1Aa, Cry1Ab, Cry1Ac or Cry2Aa in Swiss albino mice. J Hematol Thromb Dis 1(1).
10. http://endsciencecensorship.us3.list-manage.com/track/click?u=d6862ffb965b93d2616631e16&id=8996c69605&e=477dd18fb5