The outrageous behaviour of government GMO regulatory bodies around the world in trying to discredit the 2012 Seralini study continues to be exposed. The latest episode concerns CTNBio, the Brazilian commission that regulates GMOs.
Scientists from Brazil’s GMO regulatory agency protest dismissal of Seralini study
GMWatch comment
21 May 2013
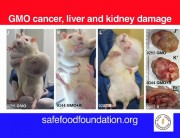
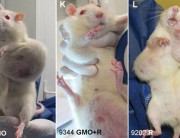
Seralini’s study found that a Monsanto GM maize, NK603, and Roundup herbicide caused organ damage and increased rates of tumours and premature death in rats.
In Brazil, four pro-GM scientists, two of whom were members of CTNBio, criticized Seralini’s study in a report of October 2012. Their report was published as the view of CNTBio as a whole, in combination with Brazil’s Ministry of Science, Technology and Innovation:
http://www.ctnbio.gov.br/upd_
But now it’s clear that no consensus existed in CTNBio. In March 2013, 15 scientist members and former members of CNTBio wrote a detailed scientific counter-report which debunks the arguments of the four pro-GM scientists’ report and supports the validity of Seralini’s findings.
The counter-report, addressed to the president of CTNBio, says that the four pro-GM scientists’ report “cannot be considered to be the position of the Commission, given that it was not evaluated by a plenary session. Even if it had been, the opinion issued by these doctors does not represent a consensus in this Commission.”
The counter-report concludes, “The study that is the object of this letter raises pertinent scientific questions about the chronic toxicity of a certain transgenic corn, NK603… In our understanding, the statistical analysis of the biochemical and biological data is sufficient to support the finding of what is called a situation of risk. Moreover, it supports the conclusions and title of the article [by Seralini et al], corroborating the clinical and anatomopathological observations and those with optical and electronic microscopy. In addition to the toxicological data provided about the long term consumption of NK603 corn, with or without the associated herbicide, the article by Seralini et al. (2012) supports questionings about the biosecurity and risk evaluation of the transgenic plants.”
CTNBio in a plenary session subsequently voted in favour of the original critique of Seralini and against the counter-report. But CTNBio and its president, Flavio Finardi Filho, stand accused by the landless peasant farmers’ movement MST of having strong ties to the biotech industry:
http://bit.ly/14sndwc (Google translation)
The Brazilian counter-report on Seralini’s study, signed by ten current members of CTNBio and five former members, is available in Portuguese here:
http://aspta.org.br/wp-
The English translation of the counter-report is here:
http://aspta.org.br/wp-
The Brazilian episode reflects what happened in Belgium, where an expert panel consulted by the Belgian Biosafety Advisory Council issued a nuanced opinion on Seralini’s study, with a dissenting minority opinion:
http://gmwatch.org/latest-
The arguments against Seralini’s study have been answered by Seralini’s team (http://www.ncbi.nlm.nih.gov/
—
—
Monsanto’s NK603 corn safety meets no consensus in Brazil
Official body rejects French study but decision was reached by vote, researchers complain
Press release: Grupo de Estudos em Agrobiodiversidade GEA, May 20 2013 http://aspta.org.br/campanha/
press-release-nk603/[Slightly edited by GMWatch; original at link above]
On September 2012, another study associating the consumption of genetically modified crops with health risks appeared in the scientific literature. Food and Chemical Toxicology published a study headed by Gilles-Eric Seralini, from the French University of Caen, showing that rats fed GM maize NK603 tolerant to glyphosate herbicides (Roundup), as well as rats exposed to Roundup alone, showed higher propensity to develop tumours. The authors thus concluded that “All treatments in both sexes enhanced large tumor incidence by 2–3-fold in comparison to our controls but also for the number of mammary tumors in comparison to the same Harlan Sprague Dawley strain”. The study provoked furore among official biosafety bodies. Besides demonstrating serious problems caused by a product already on the market, it highlighted major flaws in the risk assessment criteria used by regulators. The first large tumours, for instance, appeared in the 4th and 7th months of the study, in males and females respectively, though regulators never ask for tests longer than 3 months.
It was no different in Brazil. The Foreign Affair Ministry (MRE) asked CTNBio – National Biosafety Commission to report on the issue. Its president replied that he had nominated a special committee to answer MRE’s demand. The document produced was signed by four experts and repeats criticisms already answered by Seralini and colleagues in several interviews and in a letter to the editors published by the same journal, Food and Chemical Toxicology.
The CTNBio president’s paper was only discussed by CTNBio’s other members in April. After a hot debate, four members voted against it, stating that, given the way the rapporteurs were chosen, the document failed to consider contradictory views that emerged inside the Commission. Fourteen members were in favour of the document, although one knows that science is not made on a vote base.
On the same occasion a vote was taken on a request presented by the National Consumers Forum (FNEDC) demanding CTNBio to reassess the decision which released NK603 for commercial cultivation in the country and asking for the suspension of all seed containing this GM event. Also by a 14 to 4 vote the Commission refused the consumers’ petition.
A third debate still on NK603 took place. Fourteen members and former CTNBio members [this now seems to have grown to 15, judging by the signatures on the counter-report] presented a counter-report citing studies in support of the French group and their data and contesting the critiques they received. The counter-report also mentions different levels of rigour [applied to studies supportive and critical of GMOs], which could be understood as double standards, since a great deal of the criticism of the original study would perfectly fit the data submitted to CTNBio by the company that developed NK603. Experts say they would welcome the same rigorous standards being applied to all applications examined by CTNBio. Unless, that is, only studies showing negative impacts of GMs should be reviewed with such care. Again the debate ended with a 14 to 4 vote [against the counter-report].
The refusal to repeat a study correcting its methodological failings is a symptom of the prevalence of a belief that overcomes the scientific method, sounding more like a desire to support the technology and a dismissal of the opportunity to better understand the risks posed by GM crops.
…
An English translation of the Brazilian document in support of Séralini’s et al study can be found here: http://aspta.org.br/wp-
Media contacts:
Paulo Kageyama (English) – pkageyama@usp.br
Antonio Andrioli (German) – antonioandrioli@yahoo.com.br
Gilles Ferment (French) – gferment@hotmail.comm


































































Dear Readers.
There is no “outrageous behavior” of Brazilian CTNBio or of any other regulatory body, simply scientific disagreement of what was published by Séralini and coworkers. Indeed, the paper was discredited by many different scientific boards.
It is neither fair nor additive to put the discussion in such a ground, i.e., an arena to discredit the agencies on the basis of a hypothetical, unproven bias toward non-scientific decisions. I hope, for the improvement of knowledge and mutual understanding, that this site does not keep unduly attacking the scientific boards and agencies, but do fight for its points of view using the adequate weapons generously provided by the scientific method.